Enhancing Healthcare with Aura Care’s Medical Equipment Expertise
In the dynamic realm of medical Equipment, ensuring safety and efficacy is paramount. At Aura Care, we pride ourselves on delivering high-quality medical equipment that aligns with regulatory standards. Let’s delve into the significance of medical Equipment classes and how manufacturers like Aura Care navigate this intricate landscape.
Decoding Medical Equipment Classes: A Crucial Regulatory Framework
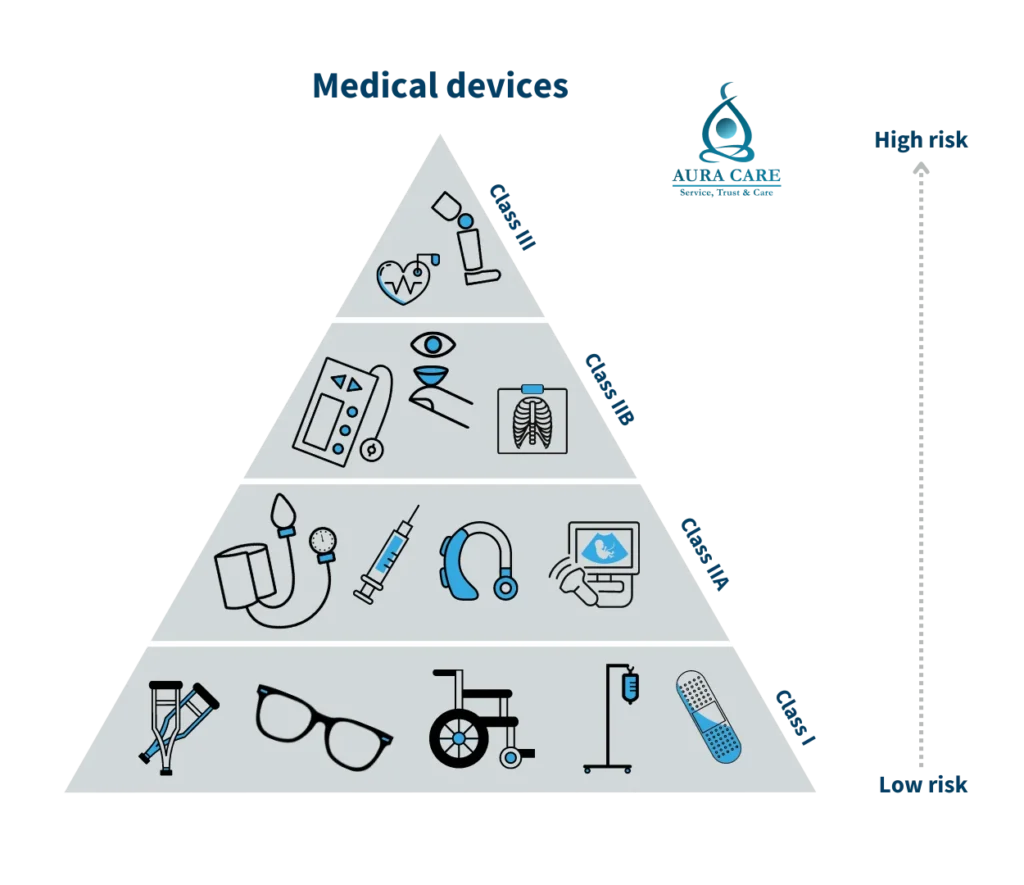
When it comes to medical equipment, regulatory agencies play a pivotal role in safeguarding patients and users. The classification system, as defined by esteemed bodies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), categorizes devices into different classes based on the associated risk. This classification is not just a regulatory formality but a key determinant of the pathway manufacturers must follow.
Aura Care’s Commitment to Safety: Understanding Device Classes
Class I: Crafting Confidence in Low-Risk Devices
Aura Care acknowledges the importance of low-risk devices, exemplified by everyday essentials like bandages and tongue depressors. These products, subject to minimal regulatory control, play a crucial role in healthcare settings. Our commitment to quality ensures that even in Class I, we adhere to rigorous standards.
Class II: Elevating Standards in Moderate-Risk Devices
Moderate-risk devices, such as powered wheelchairs and diagnostic equipment, require a higher level of regulatory scrutiny. Aura Care’s dedication to excellence is evident in the meticulous processes involved in bringing these devices to market. We navigate the regulatory landscape with precision, ensuring compliance with premarket notification requirements.
Class III: Pioneering Excellence in High-Risk Devices
For high-risk devices like implantable pacemakers and life-supporting equipment, Aura Care goes above and beyond. With stringent regulatory requirements and the need for premarket approval, our commitment to safety and efficacy stands as a testament to our dedication to advancing healthcare technologies responsibly.
Class IV (in some regulatory systems): Reserved for Exceptional High Risk
Some regulatory systems, like the European Union, have a Class IV designation for certain high-risk devices that require additional scrutiny. These devices often involve new technologies or present unique challenges in terms of safety and efficacy.
Redefining Medical Equipment Excellence
As a manufacturer committed to advancing healthcare, Aura Care understands the critical role that medical device classes play in shaping the regulatory pathway. Our active involvement in ensuring safety and efficacy sets us apart, making Aura Care a trusted name in the realm of medical equipment.
In conclusion, the classification of medical devices is not merely a bureaucratic process; it’s a commitment to excellence and a testament to Aura Care’s dedication to advancing healthcare responsibly and safely.
Explore the range of medical equipment at Aura Care and witness the difference in quality and commitment.


